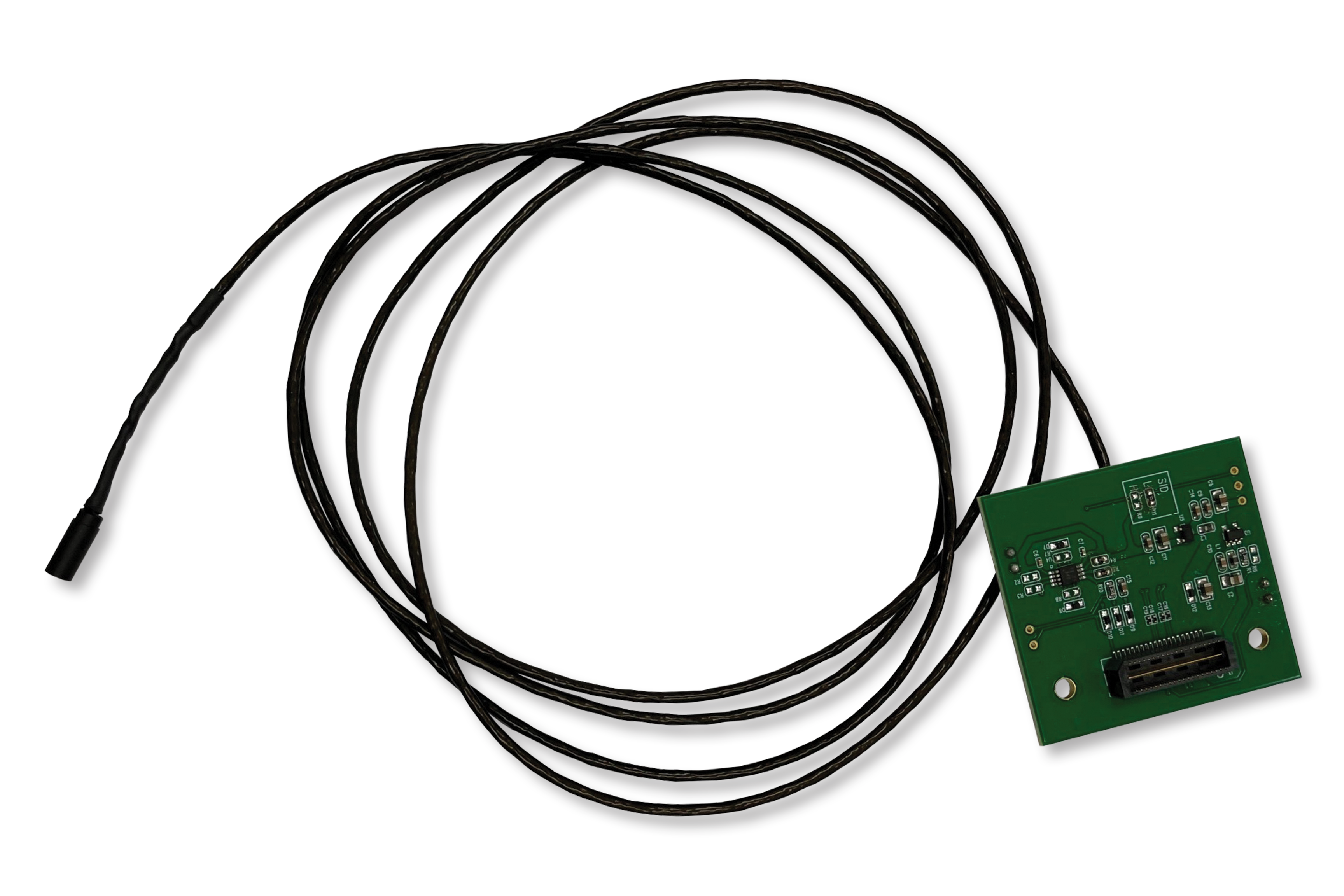
Fully-Integrated Imaging Subsystem with a Camera, Cable and Mini-LED Illumination Features Digital MIPI and Analog Outputs, Reduces Design Complexity and Cost and Speeds Time-to-Market
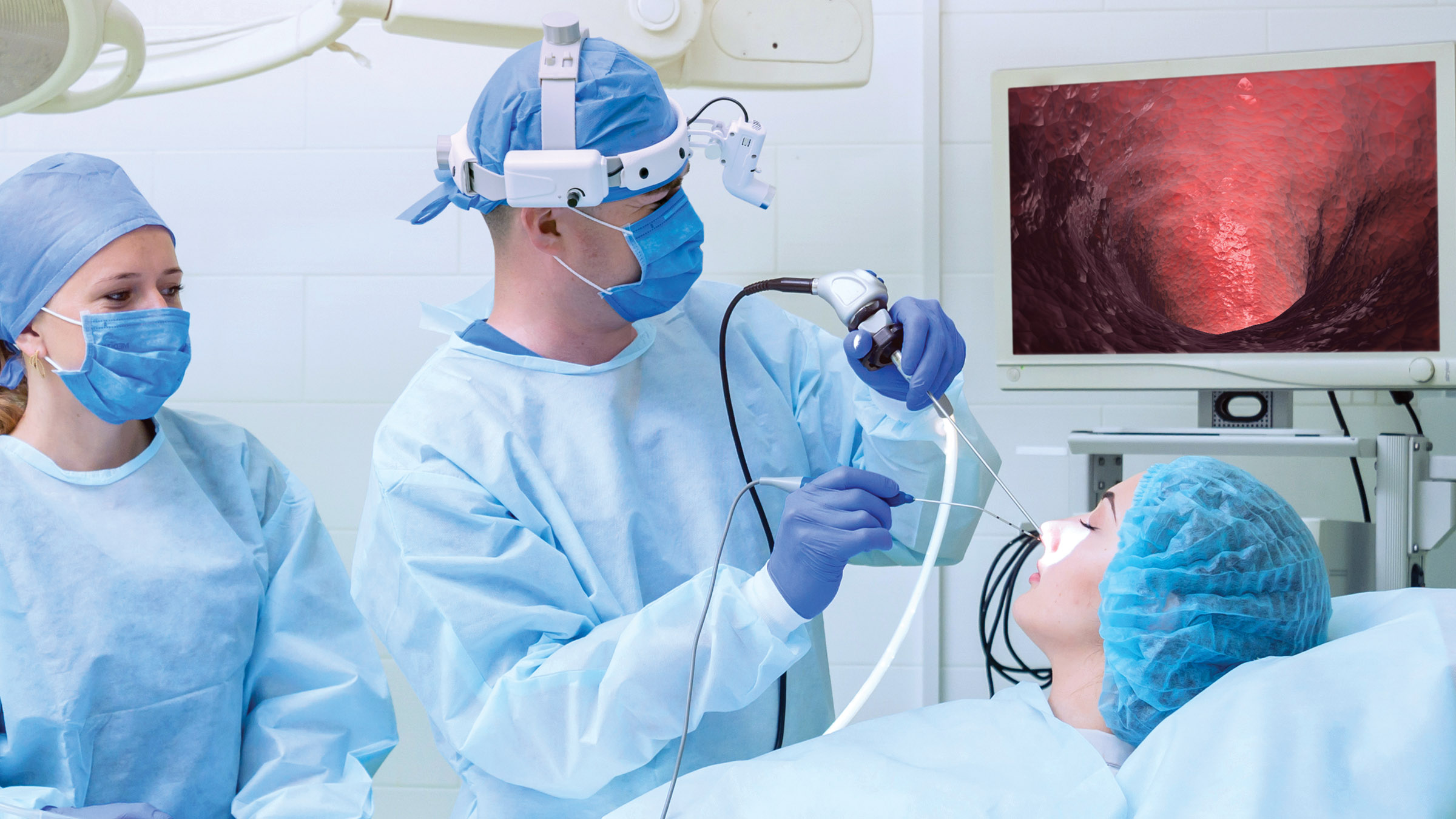
SANTA CLARA, Calif. — October 19, 2021 — OMNIVISION Technologies, Inc., a leading developer of advanced digital imaging solutions, today announced the OVMed® OCHSA and OCHTA cable modules for single-use endoscope and catheters. The fully-integrated medical-grade imaging subsystems combine an exceptionally small diameter camera module with upgraded OVMed® cables and mini-LED illumination for a complete, fully medically-tested assembly that provides excellent image quality and reduces development cost and time-to-market.
Single-use medical endoscopes will continue to expand at a rapid pace over the coming years. According to Yolé Développement, a Market Research & Strategy Consulting Company, single-use medical endoscopes will grow at a CAGR of 27 percent from 2019 to 2025, with a CMOS image sensor camera module market of US $241 million in that segment.1
“Previously, single-use endoscope and catheter designers incurred significant time and cost to separately source cables and LEDs, and then pair them with our wafer level modules,” said Ehsan Ayar, medical product marketing manager at OMNIVISION. “We are helping our customers shorten time to market with a complete, fully tested subsystem that can be integrated into single use endoscopes to allow doctors to see more detail and reach the body’s smallest anatomy.”
OVMed® OCHSA with Digital MIPI Output
The OCHSA cable module offers 800 x 800 pixel resolution with high-speed MIPI digital interface for larger outer diameter endoscopes, such as the bronchoscope, thoracoscope, pleuroscope, mediastinoscope, duodenoscope, and amnioscope. The cable module is designed to handle both short and long distances and can be customized as needed.
OVMed® OCHTA with Analog Output
The OCHTA cable module integrates OMNIVISION’s new OH0TA image sensor, which when compared with its Guinness World Record-holding predecessor, is even smaller and has quadruple the resolution. The OCHTA cable module provides wafer-level optics in a single compact assembly. With its particularly small size, the OCHTA cable module enables endoscopic devices to reach deeper into the body for a wide range of procedures including neuro, ophthalmic, ENT, cardiac, spinal, urology, gynecology, and arthroscopy procedures; it can also be used in dental and veterinary applications.
The cable modules feature OMNIVISION’s CameraCubeChip® wafer-level technology in a tiny 0.65 mm x 0.65 mm camera module, with 400 x 400 or 160 KPixel resolution for high quality image captures. They also feature the latest OVMed® cables that are highly flexible and come in a thin micro-coaxial form factor.
Integrated Mini-LED Illumination for Precise Brightness Control
The first-ever commercially-available integrated mini-LED illumination for single use endoscopy provides surgeons with precise brightness control, enabling them to clearly visualize anatomies that they couldn’t before. It features a higher contrast ratio within a smaller local area for extremely small package sizes, providing individual brightness and dimming control for multi-spectrum (RGB/IR) devices in surgical procedures.
“With these fully integrated cable modules, medical OEMs can now develop turn-key, mass-produced, high-resolution, single-use devices for deep anatomical access, while addressing the many challenges posed by reusable equipment, including cross-contamination risks and high maintenance costs,” adds Ayar.
OMNIVISION’s OVMed® cable modules are medical-grade, trusted components that undergo comprehensive certification, qualification and testing, including testing for banned substances, sterilization, biocompatibility, workmanship, operational tests and stress tests. This increases the likelihood and speed of FDA certification for medical device OEMs, while providing doctors and patients with a high level of confidence in the endoscope device.
Both cable modules are available for sampling now and are available in a flexible configuration: short (0.25 m to 3.0 m) or long (3 m to 5 m). The integrated mini-LED illumination feature will be available 1H 2022. OMNIVISION offers an evaluation kit with a pretested OVMed® image signal processor to enable rapid evaluation and development.
Please visit OMNIVISION for a demonstration during these upcoming medical device shows: MD&M Minneapolis, Nov. 3‑4, booth 1639; CompaMed, Nov. 15‑18, booth 8BH33; MD&M East, Dec. 7‑9, booth 828. For more information, contact your OMNIVISION sales representative: www.ovt.com/contact-sales.
1 Source: Status of Medical Imaging Equipment and Detectors 2020 report, Yole Développement.